Welcome to Organoboron Compounds Database
Boronic acids and their esters are highly popular synthetic intermediates in organic synthesis for their ease and efficiency of conversion to other functional groups, in metal-catalyzed cross-coupling reactions and for their unique biochemical activity.
The palladium-catalyzed cross-coupling reaction of organoboron compounds with organic halides or pseudo-halides - the Suzuki-Miyaura reaction - is a remarkably useful tool in organic synthesis.
A new class of air-stable boronic acid derivatives is trifluoroborates, which are offering a unique alternative to most boronic acids, esters and organoboranes for use in Suzuki-Miyaura and other transition-metal-catalyzed cross-coupling reactions.
As inhibitors of serine proteases, boronic acids inhibit therapeutically relevant proteases and proteasomes. FDA has approved the first boron-containing drug, bortezomib (Velcade) for the treatment of multiple myeloma and mantle cell lymphoma.
A chemical database containing CAS number, synthesis references, physical properties (melting/boiling point, refractive index, density and solubility) for more than 2,000 boronic acids, boronic acid esters and trifluoroborates is available on our website.
Search by Keyword
Please a chemical name or CAS registry number to see if that chemical is listed in our database:
Prepared Searches
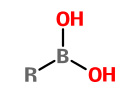 All boronic acids |
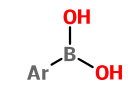 Arylboronic acids |
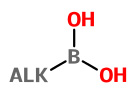 Alkylboronic acids |
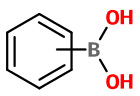 Phenylboronic acid |
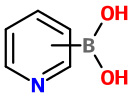 Pyridineboronic acids |
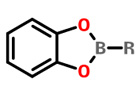 Catechol esters |
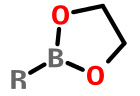 Ethylene glycol esters |
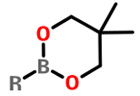 Neopentyl glycol esters |
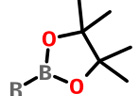 Pinacol esters |
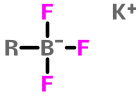 Trifluoroborate Salts |
If you have any questions regarding our website, please contact us at chem-at-organoborons-dot-com. Visit also Amino Acids and Peptides guides.